Scaling hydrogen production | The potential of Spark Ablation for single-atom catalysts
In our previous blog post, we delved into the realm of catalyst clusters, tiny titans, that offer unparalleled control over the catalyst active site, which can substantially enhance catalyst performance. Today, we will focus our attention on a very special type of electrocatalyst: the single-atom catalyst. This catalyst that shows the highest atom efficiency, meaning the capacity of each atom of active material can efficiently participate in the reaction. Furthermore, we will explore methods to enhance this atom efficiency even more, with the goal of improving the viability of green hydrogen production. The energy carrier of the future!
Navigating the hurdles: Scaling hydrogen production for sustainability
As the world gravitates towards sustainable solutions, hydrogen is increasingly recognized as a critical element in the energy landscape, poised to play a pivotal role in reducing global CO2 emissions. Its ability to act as a clean, versatile energy carrier makes it a front-runner in the race to replace fossil fuels and decarbonize various sectors of the economy.
Despite its potential, the path to integrating hydrogen into our energy systems is fraught with challenges. The core issue lies in the scalability of production processes. Current technologies, such as electrolyzers, need to transition from megawatt to gigawatt capacities to meet the burgeoning demand. This scale-up is not only a technical challenge but also a financial one, given the significant capital costs involved in electrolyzer deployment.
Iridium: The Achilles' Heel of cost-effective hydrogen production
At the heart of the cost dilemma is the reliance on iridium oxide, a catalyst critical for the anodic reaction in PEM water electrolysis. Iridium is one of the least abundant elements in the Earth's crust, leading to its exorbitant price of over €160,000 per kilogram, which is expected to significantly increase over time [1]. With typical loading in the 1-2 mg/cm2 range, it is comprised of >10% of a proton exchange membrane (PEM) electrolyzer’s CAPEX (capital expenditure) with 2019 prices.
The steep cost of iridium presents not only a challenge but also a significant opportunity for innovation. Reducing the amount of iridium required in electrolyzers could dramatically decrease the overall capital costs and pave the way for more economically viable hydrogen production. This is not just a technical necessity but a strategic imperative to enable a sustainable energy future.
Revolutionizing catalyst performance with nanotechnology
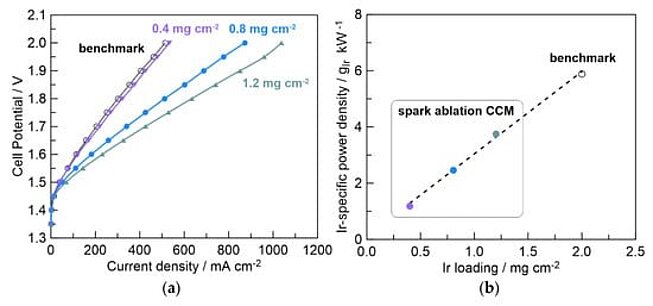
Customers of VSparticle have experienced breakthroughs in catalyst efficiency using VSP-G1 nanoparticle generator that uses the spark ablation technique to produce iridium nanoparticles.[3] These nanoparticles, just 2 nm in size, are coated onto membranes and have demonstrated a remarkable capacity to decrease iridium usage (Figure 1). Specifically, they reduce the iridium loading by a factor of four compared to traditional commercial iridium-based catalysts, all while maintaining equivalent performance levels.
The Impact of reduced particle size on catalyst activity
The key to this enhanced efficiency lies in the reduced size of the iridium nanoparticles. At merely 2nm, these particles present a greater surface area relative to their volume, which significantly boosts their reactivity. This increased catalytic activity means that less iridium is needed to achieve the same levels of performance as larger, conventional iridium catalysts. This not only represents a leap forward in terms of resource efficiency but also underscores the potential cost savings in large-scale applications. Although this is a remarkable achievement by itself, in this blogpost we are going to explore the possibility of reducing the cost even further by means of single-atom catalysts: catalyst in which the catalytic active site comprises only a single atom!
The superior efficiency of single-atom Iridium catalysts
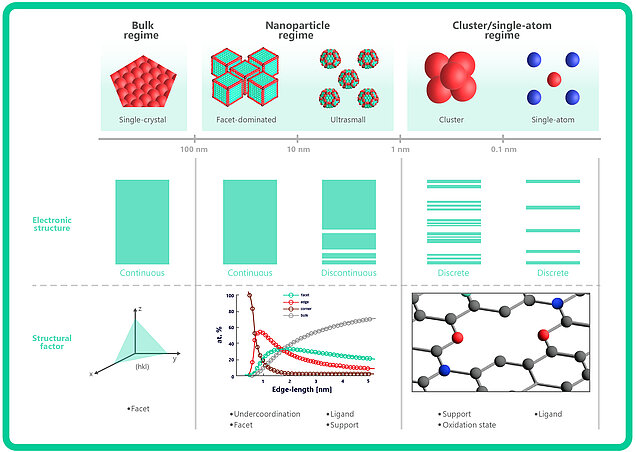
Single-atom catalysts represent the highest level of atom efficiency, redefining the capabilities of catalysts. They consist of just one active site, made up of a single atom placed on a support structure (Figure 2).[3] If the catalytic activity of iridium is determined by the element itself, utilizing a single atom as the active site, as opposed to a cluster of particles, would minimize the material required to achieve the desired catalytic performance. Furthermore, initial findings indicate that this efficiency does not come at the expense of effectiveness: single-atom iridium catalysts have demonstrated high levels of activity for the anodic reaction in PEM electrolysis, surpassing the performance typically achieved with nanoparticle-based catalysts. [4]
Pitfalls in current synthesis techniques
A significant hurdle in the production of single-atom catalysts is the inefficiency inherent in current synthesis methods. These methods, while capable of producing single-atom catalysts, embed catalysts, embed these atoms within the bulk of the material rather than positioning them at the surface where they are most effective. This misplacement results in a majority of these potent single atoms being inaccessible and unable to participate in catalytic reactions. This wastes precious metal that results in high CAPEX but reaps no benefit!
Single-atoms catalyst with Spark Ablation
VSParticle technology uses spark ablation, a technique that allows to produce nanoparticles in the aerosol. [6] It involves generating a spark that vaporizes the target material – in this case, iridium – resulting in cloud of atoms that rapidly cool and form into particles. Unlike conventional methods, spark ablation can localize these particles solely at the surfaces of substrates, enhancing their exposure and reactivity. Theoretically, if one could rapidly expose the cloud of atoms to a support, and prevent them from coalescing (forming larger particles), single-atom catalyst could be produced. This would then yield a PEM catalyst that would exhibit 100% atom efficiency! The question is, is it technically feasible?
From particles to clusters to single atoms
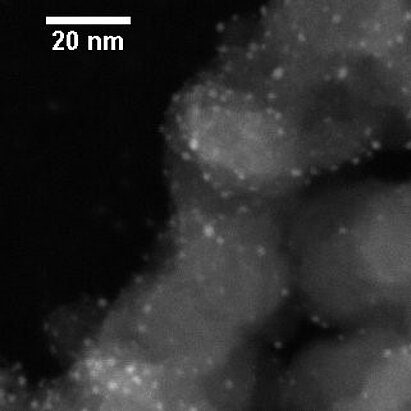
Recently, VSParticle customers have shown that the size of the particles produced with spark ablation can be reduced all the way down to a few tens of atoms.[7] Further, they can be immobilized on supports without agglomeration and used in electrolysis (Figure 3). This is 100 times less atoms per catalyst particle compared to previous standards, bringing them remarkably close to generating of single-atom catalysts. This would suggest it is technically feasible! For those of you interested in cluster catalysts, you can find some further reading here.
VSParticle's Nanoprinter: Revolutionizing synthesis of (semi-)conductive oxides supported catalysts.
VSParticle is a pioneer in the field of (nano)particle generation and printing. The company’s flagship product, VSP-P1, enables fast and easy synthesis and printing of (semi-) conductive nanoporous layers for various applications.
VSP-P1 nanoprinter is fully equipped to produce (semi-)conductive oxide supports relevant as catalyst supports. [8] The combination of the nanoprinter with two VSP-G1 sources allows for the sequential printing of a support layer followed by a catalyst layer. Alternative, co-production of support and catalyst layer using a co-deposition configuration offers further flexibility in catalyst design. [9]
Stay tuned for our next blog post where we dive into vast configurational space of high-entropy alloys and how AI informed closed loop optimization schemes will be needed to harvest their full potential.
Acknowledgment
Special thanks to Cedric David Koolen, Principal Investigator @EPFL, for sharing his knowledge and expertise.
References
[1] https://pmm.umicore.com/en/prices/iridium/
[2] Mayyas, A. T., Ruth, M. F., Pivovar, B. S., Bender, G. & Wipke, K. B. Manufacturing Cost Analysis for Proton Exchange Membrane Water Electrolyzers. www.osti.gov/biblio/1557965 (2019) doi:10.2172/1557965.
[3] Koolen, C. D., Luo, W. & Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 948–973 (2022) doi:10.1021/acscatal.2c03842.
[4] Zhu, Y. et al. Iridium single atoms incorporated in Co3O4 efficiently catalyze the oxygen evolution in acidic conditions. Nat. Commun. 13, 7754 (2022).
[5] Schmidt-Ott, A. Spark Ablation: Building Blocks for Nanotechnology. (Jenny Stanford Publishing, 2020).
[6] Scalable synthesis of Cu(-Ag) oxide clusters via spark ablation for the highly selective electrochemical conversion of CO2 to acetaldehyde. www.researchsquare.com (2024) doi:10.21203/rs.3.rs-3791391/v1.
[7] Drdova, S. et al. Precursor- and waste-free synthesis of spark-ablated nanoparticles with enhanced photocatalytic activity and stability towards airborne organic pollutant degradation. Environ. Sci. Nano 11, 1023–1043 (2024).
[8] Tiwari, A. et al. X-ray standing wave characterization of the strong metal–support interaction in Co/TiOx model catalysts. J. Appl. Crystallogr. 57, 481–491 (2024).
*The publication: ‘Scalable synthesis of Cu(-Ag) oxide clusters via spark ablation for the highly selective electrochemical conversion of CO2 to acetaldehyde,’ is a preprint and has not yet undergone peer review. Therefore, its findings are provisional, and its conclusions may change.
Comments
No Comments